
You are:
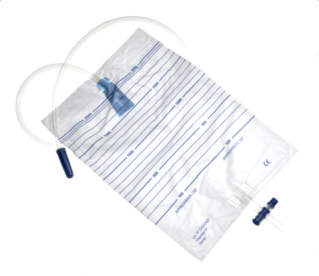
The Colva urine bag enables short-term urine drainage.
Its connector adapts to all urinary catheters so there is no risk of incompatibility.
The check valve prevents any urinary infection. Its 4 reinforced eyelets adjust to all types of hooks
Indications
Indicated for short-term urinary drainage (maximum 24 hours of use or as per the prescribing practitioner's recommendations, or according to hygiene protocol)
Used materials
PVC: Bag, tube, catheter fitting - PE: Cap
Labeling
on each unit are written the reference, the lot/serial number, the sterilization method and the expiry date if applicable
medical device
sterile
conformity
economic operator
Transport conditions
Protected from light, humidity and heat
| Conditioning | Parts quantities | Dimensions in cm | Weight in kg | Ean 13 of conditioning |
| UNIT | 1 | 3661809003660 | ||
| BAG | 25 | 3661809103667 | ||
| CASE | 250 | 38 x 30 x 31 | 9.550 | 3661809203664 |