
You are:
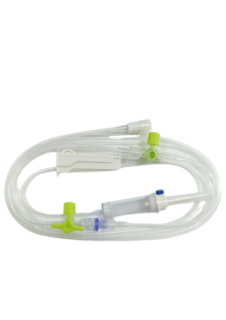
The sterile, DEHP-free INFINEED 3-way infusion set with non-return valve has been designed to minimize its environmental impact, using only DEHP-free raw materials (eliminating components containing DEHP). The polycarbonate-free 3-way stopcock has been tested at pressures well in excess of those recommended for gravity infusion; laboratory tests have shown a pressure resistance of around 10 bar. Contact with 0.5% or 2% Chlorhexidine in 70° Isopropyl alcohol, without evaporation time, while the luer lock is connected, and therefore under physical stress, presents a significant risk of luer lock cracking, and should be avoided wherever possible. In line with SF2H (2019) recommendations, disinfection of connectors (when necessary) with 70° ethyl alcohol is always preferable, as it is just as effective in disinfecting inert materials and with much less risk of altering plastics.
The non-return valve is a one-way valve which acts as a safety device (particularly when combining gravity and pressure infusion) by preventing any backflow of solution into the bag or vial. It has a return pressure resistance of 3 bar.
Perforator available:
- Perforator: Bicanal with gripping wings.
- Air intake: Supplied closed for immediate use on bag (except for infusers with non-return valve) - 0.5µm filter.
- Chamber: Flexible and translucent, the chamber is calibrated to 20 drops = 1ml ± 0.1ml. 15µm anti-particle filter.
- Wheel: Precise and reliable, linear flow regulator.
- Tubing: PVC, DEHP-free, designed for consistent adjustment. Tube diameter: 3 x 4.1mm.
- End cap: Luer Lock mobile.
- End cap: 1µm purge filter with antibacterial membrane.
- Bisphenol A-free 3V tap.
- Polycarbonate and silicone non-return valve.
Tube dead volume: 12.50ml.
Chamber dead volume: 5.10ml.
Made in China.
Indications
This device enables gravity administration of injectable parenteral preparations such as drugs, solutions and parenteral nutrition.
It is particularly recommended for mixed mechanical and gravity infusion: the non-return valve must be fitted to the gravity line.
Gravity infusor, not to be used for pressurized infusion, notably injection of contrast media. Not to be used for transfusion, not to be used for drugs requiring a 0.2µm filter.
Dispose of in accordance with local regulations.
Used materials
PVC: Chamber, air intake, DEHP-free tubing.
Polyethylene: Purge filter, cap, perforator protector.
Acrylonitrile Butadiene Styrene: perforator, mobile Luer Lock, linear flow regulator.
Nylon: Particle filter.
PC: Non-return valve.
Polypropylene: Protective tap caps.
High density polyethylene HDPE: Faucet key.
BPA-free Tritan ® copolymer: Faucet body.
Silicone: Lubricant, tap, non-return valve.
Labeling
on each unit are written the reference, the lot/serial number, the sterilization method and the expiry date if applicable
medical device
sterile
conformity
Transport conditions
Protected from light, humidity and heat
| Conditioning | Parts quantities | Dimensions in cm | Weight in kg | Ean 13 of conditioning |
| UNIT | 1 | 06972620110923 | ||
| BAG | 25 | |||
| CASE | 200 | 49 x 58 x 35 | 16972620110920 |