
You are:
Rotary flow regulators have a standardized flow stability of +/- 10% over time (ISO8536-14 standard), and are therefore considered lt;precision lt; regulators. It should be noted that many factors influence flow regulation (viscosity, height, blood pressure, etc.): drop-counting and regular monitoring are therefore good practice when using these precision regulators. - After purging the line, and before connecting the device to the patient's vascular access, turn the flow regulator to the lt;off lt; position. Contact us to obtain the good use sheet.
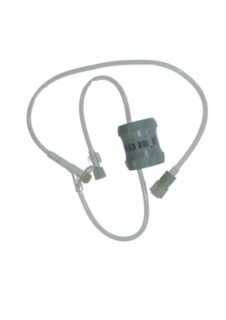
Extender with :
High-quality tubing: flexible, anti-plication PVC
Precision rotary flow regulator: Smooth, easy adjustment - Constant, reliable flow
Optimum technical features: Movable Luer Lock tip, Graduations from 0 to 250 mL/h.
Nonpyrogenic device.
Pressure resistance: 50 KPA (0.5 Bars - 7.25psi)
Dead volume: 3.08ml.
Made in China.
Purging information: graduations are reliable to +/- 20% from 20ml upwards, provided the following purging protocol is followed :
- Purge the line while keeping the flow regulator in the lt;open lt; position (delivered in the lt;open lt; position).
Contact with 0.5% or 2% Chlorhexidine in 70° Isopropyl alcohol, without evaporation time, while the luer lock is connected, and therefore under physical stress, presents a significant risk of luer lock cracking, and should be avoided wherever possible.
In line with SF2H (2019) recommendations, disinfecting connectors (when necessary) with 70° ethyl alcohol is always preferable, as it is just as effective at disinfecting inert materials, and with much less risk of altering plastics.
Indications
This device enables precise gravity administration of injectable parenteral preparations: drugs, solutions, parenteral nutrition.
Do not use with high-risk drugs, drugs with narrow therapeutic margins (catecholamines, morphine, heparin, insulin, potassium chloride, anticancer drugs requiring infusion pump administration, and all pharmaceutical specialties whose R.C.P. requires infusion pump or syringe pump administration or the presence of a 0.2µm filter).
Source : OMEDIT CENTRE -- Les bonnes pratiques de perfusion : Module lt; La précision du débit de perfusion lt;.
For peripheral lines, the infusion line can remain in place for a maximum of 96 hours (i.e., the maximum time the peripheral catheter can be in place).
For central lines, the infusion line must be changed at least every 72 hours (or according to the establishment's validated protocol).
Dispose of in accordance with local regulations.
Used materials
Cap: PE
Female Luer Lock: PC
Tube: PVC
Flow regulator: ABS / TPE
Luer Lock male: ABS
Cap: PE
Labeling
on each unit are written the reference, the lot/serial number, the sterilization method and the expiry date if applicable
medical device
sterile
conformity
Transport conditions
Protected from light and humidity
| Conditioning | Parts quantities | Dimensions in cm | Weight in kg | Ean 13 of conditioning |
| UNIT | 1 | 21 x 13 x 0 | 0.017 | 06972620110503 |
| BAG | 25 | 55 x 30 x 5 | 0.440 | |
| CASE | 150 | 39 x 42 x 31 | 3.520 | 16972620110500 |
| PALLET | 3000 | 120 x 80 x 173 | 88.400 |